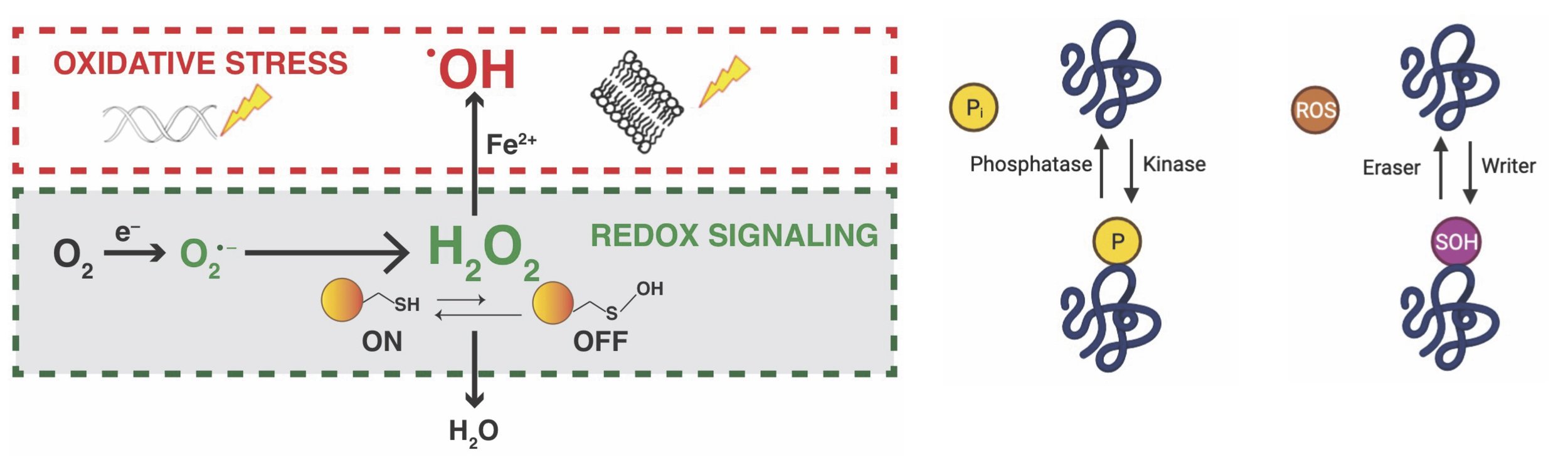
While there is a lot of interest in understanding how redox control relates to cancer, we still don't fully understand the direct effects of fluctuating levels of Reactive Oxygen Species (ROS) in cancer cells. ROS includes different molecules like the superoxide anion, hydroxyl radical, singlet oxygen, and hydrogen peroxide (H2O2). Among these, H2O2 is considered the main ROS in controlling biological activities. Interestingly, hydrogen peroxide, similar to other protein modifiers like kinases, ubiquitylases, and acetyltransferases, can affect various cellular processes. It does this by oxidizing specific amino acid residues (cysteine and methionine) in important signaling proteins. Additionally, the outcomes of redox signaling are similar to kinase signaling, providing information about the source, specificity, and consequences of the oxidative modification involved. Each redox signaling pathway also has unique regulatory mechanisms that affect its structure and timing. This complex system makes studying redox biology challenging, especially because there is a lack of accurate analytical methods to measure levels of oxidants like hydrogen peroxide and antioxidants like glutathione. These methods are crucial in tracking changes in the redox state throughout the proteome. In our lab, we use genetic and chemical proteomic techniques to explore the role of ROS and redox signaling in the development of cancer. Our goal is to contribute to the development of more effective treatments.
A pressing question in public health is whether antioxidants help prevent or promote cancer. While historically, antioxidants have been considered as cancer-preventing agents, new evidence suggests that they might actually support tumor growth. This conflicting view could be due to the fact that reactive oxygen species (ROS) are not random, but rather act as selective and diverse signaling molecules with unique functions.
Our lab investigates individual redox switches in pancreatic ductal adenocarcinoma (PDA) cells and the surrounding tumor environment to better understand their roles. Our goal is to explore the possibility of targeting specific redox dependencies in PDA cells as a therapeutic approach, aiming to eliminate tumor cells while leaving normal tissues unharmed.
More about our work:
https://cancer.columbia.edu/new-study-illuminates-key-building-blocks-pancreatic-cancer
http://cancerdiscovery.aacrjournals.org/content/6/9/945.2
https://aimsci.com/ros/index.php/ros/article/view/61
http://www.sciencedirect.com/science/article/pii/S1471491417300436
http://www.cell.com/trends/molecular-medicine/abstract/S1471-4914(17)30043-6